In terms that are widely accepted, Integrated Pest Management (IPM) can be defined as an approach to economically manage pest damage with the least possible hazard to non-target species, the environment and property. As a practical matter, IPM programs seek to attain these goals by selecting non-pesticidal control and, when such controls are unavailable or insufficient, by selecting those chemical controls which pose the lowest risk of adverse effects.
The European Community’s interest in the development and implementation of Integrated Pest Management is not new, but dates back to the first European IPM task force – the “Working Group for Integrated Plant Protection in Fruit Orchards” which was established by the International Organisation for Biological and Integrated Control of Noxious Animals and Plants (IOBC) in 1959.
Today, the European Union is at an important junction in the evolution of pest management and pesticide regulation. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 (the Framework Directive) established a framework for action to achieve the “sustainable use” of agricultural pesticides. It states: “The application of general principles and crop and sector-specific guidelines with respect to integrated pest management by all farmers would result in a better targeted use of all available pest control measures, including pesticides. Therefore, it would contribute to a further reduction of the risks to human health and the environment and the dependency on the use of pesticides. Member States should promote low pesticide-input pest management, in particular integrated pest management, and establish the necessary conditions and measures for its implementation.”
The Framework Directive requires Member States to develop, and to submit by 14 December 2012, National Actions Plans describing how they ensure the implementation of the principles of integrated pest management, with priority given wherever possible to non-chemical methods of plant protection and pest and crop management. Member States are instructed to take all necessary measures to encourage professional users of pesticides to switch to practices and products with the lowest risk to human health and the environment among those available for the same pest problem.
To the extent that these National Action Plans will allow the use of pesticides, the Plans should provide for the means to accurately and reliably assess the risks attending the use of specific pesticide products and to compare the risks associated with the use of various alternative formulations. While the Framework Directive specifically addresses the use of pesticides in agriculture, the application of IPM principles in non-agricultural settings, such as the home and garden, schools and the workplace is also dependent upon comparison of risks associated with the use of various alternative pesticide products. Therein lies a significant problem.
Pesticide products, as they are available in the marketplace, are formulations of two general categories of ingredients. “Active” ingredients are those which are included for their direct action on the target pest. The active ingredients are formulated with additonal ingredients that serve in a variety of ways to enhance and / or preserve the efficacy of the active ingredients. In the European market, the latter ingredients are known by a variety of names, including “adjuvants,” “co-formulants” and “synergists.” In the US, they are “inert” or “other” ingredients. For ease of discussion, we refer to all of these non-active ingredients as “other ingredients.” “Other” ingredients generally comprise the bulk of pesticide products and in many formulations account for more than 99% of the formulation as marketed. Under current regulatory schemes “other” ingredients present a dilemma to those who would like to make meaningful assessments of the risks of pesticide use.
First, with few exceptions, the identities of the “other” ingredients are not disclosed on the product label nor are they otherwise readily available to the consumer, be that an agribusiness or a homeowner. In Ireland, package labeling regulations require the identification of only those “other” ingredients whose toxicity and concentration exceeds prescribed thresholds. In the US, Environmental Protection Agency regulations currently require label disclosure of a scant few chemicals based on their known toxicity but independent of their concentration in the formulation. The secrecy surrounding the composition of pesticide products is inconsistent with the ingredient information readily available to consumers on product labels of other formulated items such as processed foods, drugs, cosmetics, and personal care products.
Nondisclosure of the identity of “other” ingredients is a particular concern given mounting scientific evidence that many “other” ingredients may have significant adverse consequences for human health or the environment. “Other” ingredients can increase the ability of pesticide formulations to affect significant toxicologic end points, including developmental neurotoxicity, genotoxicity, and disruption of hormone function. They can also increase exposure by increasing dermal absorption, decreasing the efficacy of protective clothing, and increasing environmental mobility and persistence. “other” ingredients can increase the phytotoxicity of pesticide formulations as well as the toxicity to fish, amphibians, and microorganisms.
Research has also shown that commercial pesticide products (including both active and “other” ingredients) have effects that cannot be accurately predicted by using data about active ingredients alone. For example:
– A dicamba-containing herbicide caused three times more damage to ovary cells than did dicamba alone.
– Absorption through the skin of a permethrin-containing insecticide was 4 – 10 times greater than for permethrin alone.
– A glyphosate-containing herbicide was more toxic to a non-target aquatic plant (Lemna gibba) than was glyphosate alone.
– A permethrin-containing insecticide caused complete mortality of developing tadpoles at a concentration of 9 parts per billion; a similar concentration of permethrin alone did not cause mortality.
– Mortality of zebra finches following exposure to a fipronil-containing insecticide was caused by a solvent (diacetone alcohol) in the product rather than the active ingredient.
– An imidacloprid-containing insecticide caused twice as much mortality of Daphnia magna as did equivalent concentrations of imidacloprid alone.
Over the years a wide variety of tools have been developed to analyze the hazard and exposure characteristics of pesticides for various potential human health and environmental impacts. As a group, these tools are generally known as “Pesticide Risk Indicators” (PRI). PRIs vary in scope and format, and may consider impacts such as toxicity to humans, birds, fish or beneficial insects and pollution of surface waters, groundwater and air. In some instances, multiple impacts may be considered and an overall rating developed.
More than 100 PRIs have been developed worldwide and at least several are in widespread use in the European Union. They have been applied to prospective assessments of pesticide impacts, as in the design of pest management programs and the preparation of environmental regulations and environmental impact assessments, to monitoring the impacts of agriculture and pesticide policies, and in the evaluation of ongoing pest management programs. Pesticide Risk Indicators vary in the range and type of pesticide attributes they include in their analysis – some focus purely on toxicological risk, while others consider the transport and fate of the applied chemicals. Most PRIs aim to produce a simplified metric or ranking system to facilitate comparison of risks associated with pesticide use and to better inform product selection.
Given the secrecy surrounding the identity of “other” ingredients in pesticide formulations, it can be argued that PRIs cannot provide accurate and reliable assessments of the risks associated with specific pesticide products as they are available in the marketplace for sale and use. Although the identity of “other” ingredients is generally not available to anyone other than the manufacturer / formulator and the appropriate regulatory authorities, there are at least a few exceptions to this rule. In a few cases, the identity of one or more “other” ingredients in specific pesticide products sold in the US has been provided in Material Safety Data Sheets, in response to inquiries to the manufacturer and as a result of legal action. With our colleague, Madison Condon, we have compared the results of PRIs applied to some active ingredients with the results of the same assessment applied to “other” ingredients with which those active ingredients are formulated in actual pesticide products. The results are disturbing.
For example, one simple PRI, the Groundwater Ubiquity Score (GUS), is an indicator of the potential for groundwater contamination based on the carbon adsorptivity (KOC) and soil half-life (DT50) of chemicals assessed. Data for those properties are available for many chemicals and enable a straightforward comparison of several pesticide active ingredients and the “other” ingredients with which they have been formulated. Based on the quantitative assessment, GUS assigns chemicals to one of 3 categories: non-leacher, transitional or leacher. In Table 1 below, the GUS evaluation of the herbicides Glyphosate, Imazapyr, 2,4-D and Alachlor are compared to the results for several “other” ingredients with which they have been formulated in some pesticide products.
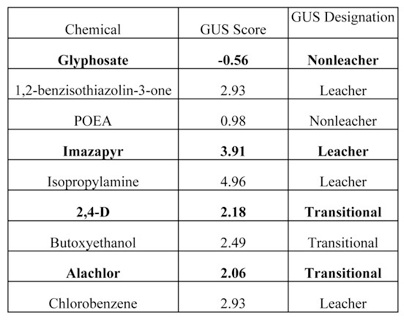
In the cases of Glyphosate and Alachlor, “other” ingredients prove to be a greater threat to groundwater than the active ingredients with which they are formulated. In these instances, a GUS analysis of the active ingredient alone, as is generally done, would provide a deceptively favorable assessment for the formulated pesticide product. The analyses for Glyphosate products show that an assessment for one formulation cannot be taken to reflect the impacts of a different product with the same active ingredient. Some glyphosate products have contained POEA but not 1,2-benzisothiazolin-3-one while other products contained both. The assessment must be product specific and must consider all ingredients in the product.
A more complex PRI, the Environmental Impact Quotient, factors the physical, chemical and toxicological properties (human, avian, fish and beneficial arthropod species) of pesticide active to derive separate Farmworker, Consumer and Ecology scores as well as a Total EIQ score for specific active ingredients. Like the GUS analysis, the score is derived for the active ingredient alone, regardless of the “other” ingredients with which it may be formulated. This total score can then be multiplied by the pesticide’s application rate to determine an EIQ Field Use Rating. The EIQ is particularly user-friendly; scores are calculated and published for individual active ingredients. The user simply consults a table to obtain the score for an active ingredient of interest. The potential for adverse effects varies with the EIQ.
Using data available for some of the “other” ingredients found in formulation with Glyphosate, Imazapyr and 2,4-D we determined their EIQs using the same calculations as the original authors used for the active ingredients. (See Table 2.) We were unable to calculate EIQs for each of the “other” ingredients that had been identified due to a lack of published data on their environmental and toxicological properties.
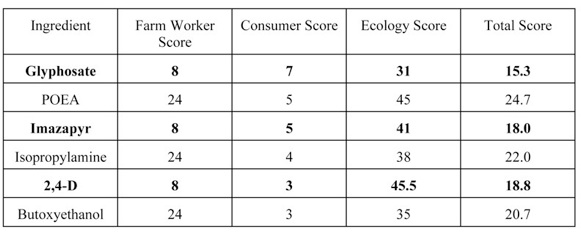
It is clear from these analyses that each of the “other” ingredients has a greater potential (higher score) to cause adverse health impacts to farm workers than their co-formulated active ingredients. POEA also has a higher “Ecology Score” than glyphosate, the active ingredient with which it is formulated. While the “Consumer Score” for the “other” ingredients was the same or lower than the “Consumer Score” for their co-formulated active ingredients, the “Total Score” for the “other” ingredients is consistently greater than that of their co-formulated active ingredients. Used as intended, the published EIQ scores may not fairly represent the potential adverse health and environmental impacts of formulated products containing the specified active ingredients.
The laws and regulations which currently enable pesticide registrants to maintain secrecy in regard to the “other” ingredients in pesticide products are, in large part, beyond the control of the research community which has developed the PRIs, beyond the control of professional risk assessors and beyond the control of the non-scientific communities which may depend on PRI evaluations to determine pest management policies and practices. Without full public disclosure of the identity of all ingredients formulated in pesticide products, active and “other” ingredients alike, accurate and reliable risk assessments will not be possible. Without a means to compare risks, there is little basis to select the lowest impact pesticide decisions that are at the heart of any IPM program.
With National Action Plans due to be submitted in just over 2 years, now is the time for action. Steps should be taken to revise existing pesticide regulations, as they apply to both agricultural and non-agricultural pesticide products, to insure full public disclosure of all ingredients. Ideally, that information should be available to the consumer prior to the time of purchase, on the package label. Litigation and/or legislation may be required to force such full disclosure. In the US, the Environmental Protection Agency has undertaken a rulemaking process to consider options for increasing the public disclosure of inert ingredients. That process was initiated in response to petitions submitted by the Attorneys General of several states and by a large number of citizens’ advocacy groups.
In addition to full disclosure of the identity of “other” ingredients, it may also be necessary to expand the data required from pesticide registrants to assure that they adequately characterize the physical, chemical and toxicological properties of “other” ingredients and of the formulated products. Much of this data is not currently required by the pesticide registration process. If such information were available it would be a relatively simple matter to modify PRIs. Until such time as that information is available, PRIs should be used with extreme caution and with full disclosure of their limitations. The selection of non-pesticidal control methods remains the most certain means of assuring the lowest potential for adverse effects.
Michael Surgan retired after 28 years of service as the Chief Scientist at the New York State Attorney General’s Environmental Protection Bureau.
Caroline Cox is the Research Director for the Center for Environmental Health in Oakland, California.
Further Reading:
Cox, C. and M. Surgan, 2006. Unidentified Inert Ingredients in Pesticides: Implications for Human and Environmental Health. Environmental Health Perspectives 114(12):1803 – 1806.
Surgan, MH, M. Condon and C. Cox, 2010. Pesticide Risk Indicators: Unidentified Inert Ingredients Compromise Their Integrity and Utility. Environmental Management 45:834–841.
United States Environmental Protection Agency, Advanced Notice of Proposed Rulemaking, Public Availability of Identities of Inert Ingredients in Pesticides. Fed Reg 74(245), December 23, 2009. Available at edocket.access.gpo.gov/2009/E9-30408.htm.


No comments yet, add your own below